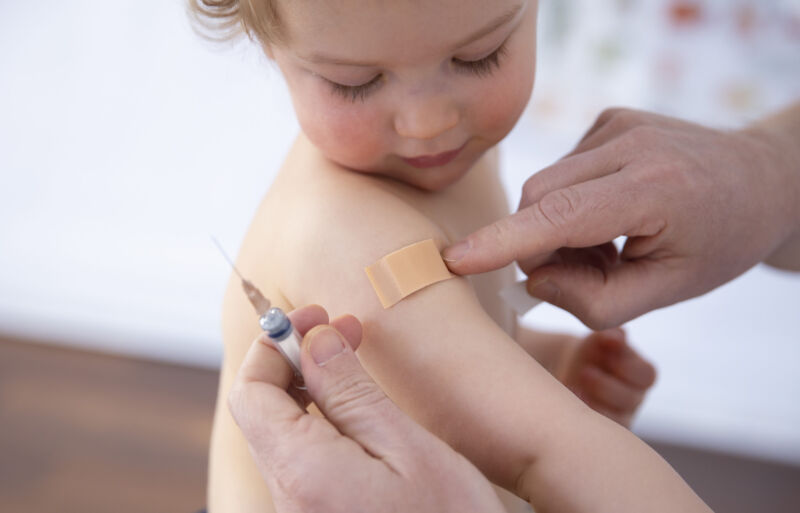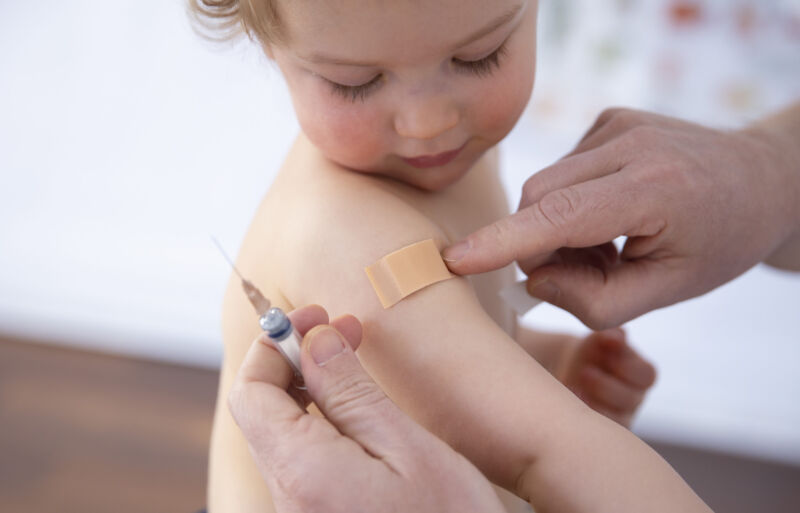
Enlarge / A child getting a vaccination on February 19, 2021, in Bonn, Germany. (credit: Getty | Ute Grabowsky)
The Food and Drug Administration may be reconsidering its criteria for authorizing COVID-19 vaccine doses for children under age five, according to Scott Gottlieb, a former FDA commissioner and a current board member of vaccine-maker Pfizer. This opens the possibility that vaccine-ineligible youngsters could get protection from severe COVID-19 sooner than anticipated.
In an interview Sunday, Dr. Gottlieb told CBS’s Face the Nation that he sensed a shift in federal health officials’ thinking on the younger group. “And I’m hopeful that you could see some movement on trying to entertain that application earlier,” he said. “Ultimately, the decision resides with FDA, but there is some indication that there may be an early reaction on that application.”
If Gottlieb’s inkling is correct, vaccines could begin going into little arms as soon as March.