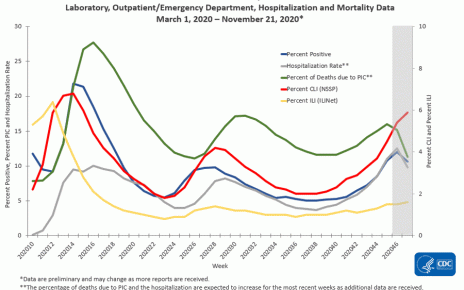
The mad dash to produce and produce a COVID vaccine isn’t really over. However, the start of the ending might be someplace in sight.
By late September, four large scale, late-stage clinical trials had been detained to get a COVID-19 shot at the USA. Included in these are a candidate out of Pfizer and German spouse BioNTech (BNT162); Johnson & Johnson medication arm Janssen’s very own experimental treatment (JNJ-78436725); 1 from biotech Moderna (mRNA-1273); along with British drugmaker AstraZeneca’s AZD1222.
“That is an unprecedented effort to the scientific community made possible by years of advancement in vaccine technologies along with also a coordinated, strategic approach across industry, government, and academia.”
{However, the path to creating a vaccine isn’t simple and includes the dangers of the {} , according to a Food and Drug Administration (FDA) temporary stop on Johnson & Johnson’s and AstraZeneca’s Stage III studies. |} Those research have now resumed following a security commission determined that unwanted effects and disorders in a few research participants were irrelevant to the vaccines.
These trials have been enormous undertakings, and also the firms are quickly reaching, should they harbor ’t {} , their enrollment objectives. For example, the past week Moderna declared it’d completed registration of 30,000 individuals in its own trial. Pfizer said during a sales call on Tuesday it had registered 42,000 individuals, 36,000 of whom have {} the required second dose of this vaccine. The business intends to register another 2,000 participants.
AstraZeneca and Johnson & Johnson have resumed enrollment following the temporary stop, and the firms have stated that they ’re planning to recruit around 30,000 adults from the U.S. and 60,000 individuals in numerous nations , respectively.
The coming weeks and months will be crucial for these businesses jockeying to be {} the COVID race. Even though Pfizer CEO Albert Bourla has said that interim information on how successful its offender will come before Novemberhe confessed on Tuesday it’s {} to come before the next week of November.
If the information prove promising, then the business could submit an application to get an FDA emergency use authorization (EUA) when the fourth week of November.
“And {} up to the FDA to analyze the document and choose whether they’re likely to provide us consent and the length of time it could take them through this document,” Bourla stated during a meeting in the Fortune International Forum virtual convention on Tuesday.
Other businesses might take more since the FDA has various prerequisites before taking applications for emergency usage, such as following up with patients for 2 weeks to ensure that the vaccines are secure prior to submitting a filing.
And even after a product comes with an emergency consent, which might require at least twice to three months following the FDA has obtained a drugmaker’therefore program it wouldn’t be accessible for bulk commercial supply for just anybody. It would probably take before the middle of the this past year before that’s an opportunity, whilst in the meantime an expected vaccine granted emergency consent would probably be hauled into the highest-risk inhabitants.
Much more coronavirus policy out of Fortune:
- The way the funeral motivated the pandemic’s latest hardware
- U.S. countries are turning into a private Irish business to help block the spread of COVID
- “A story of two Americas”: The pandemic is Growing the fiscal wellness difference
- Procter & Gamble indicates that raising spending in a downturn is well worth it
- May COVID-19 induce diabetes? Here is what we understand