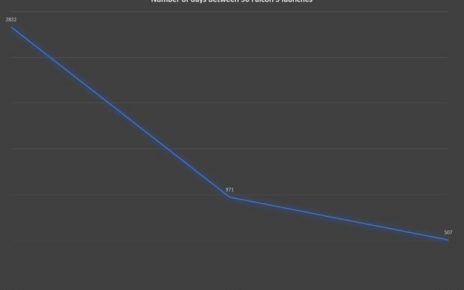
Enlarge / Vials with COVID-19 Vaccine labels showing logos of pharmaceutical company Pfizer and German biotechnology company BioNTech. (credit: Getty | Photonews)
A committee of independent advisors for the Food and Drug Administration has voted unanimously (18 to 0) in favor of authorizing a booster dose of the Pfizer/BioNTech COVID-19 vaccine for people aged 65 and older, as well as people at high risk based on an underlying medical condition and/or occupational exposure (e.g., healthcare workers). The booster doses are recommended to be given at least six months after completion of the primary two doses.
If the FDA moves forward with the advisory committee’s recommendation—which it likely will—boosters will be offered to those two groups based on an Emergency Use Authorization.
Prior to voting in favor of authorization for the two groups, the committee rejected the idea of approving boosters for all people ages 16 and up with a resounding vote of 16 to 2 against.