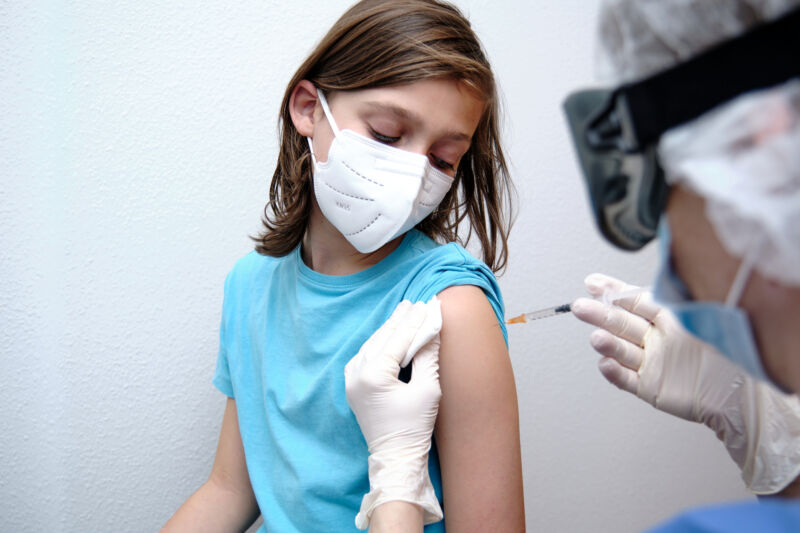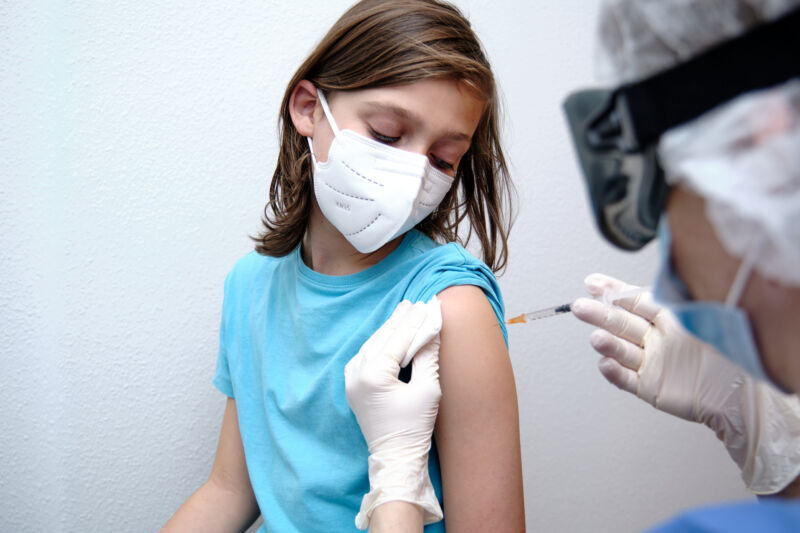
Enlarge / With new data, we’re able to expand vaccinations to ever-younger populations. (credit: Roberto Jimenez Mejias / Getty Images)
On Wednesday, the CDC’s Advisory Committee on Immunization Practices recommended that the CDC approve the use of the Pfizer/BioNTech COVID-19 vaccine for the 12- to 15-year age group. The decision comes two days after the FDA granted an emergency use authorization for the same age group and will help the US further limit the pool of people who can spread infections or foster the evolution of new viral variants. Formal CDC approval could come quickly, given recent history.
Given the FDA’s earlier decision, the move might seem anticlimactic. But having the FDA and CDC officially on the same page is reassuring, and several state-run vaccination programs are awaiting the CDC’s OK before expanding into that age group. Private providers and insurance companies were also varied in their response to the FDA’s decision and were waiting for the CDC.
The data that supported the approval was pretty decisive, as a small Phase III clinical trial of 2,260 adolescents saw 16 cases of COVID-19, with every single one occurring in the placebo group. Side effects were similar to those experienced by older people, with a brief period of flu-like symptoms. The committee was tasked with considering whether the benefits outweighed the risks; given the minor side effects and the increasingly obvious benefits of vaccination, it’s not a surprise that the vote in favor of approval by the committee was 14 in favor, none opposing, and a single recusal. The CDC director, Rochelle Walensky, is overwhelmingly likely to follow the committee’s recommendation, most likely before the day is over. (We’ll update this story if and when this occurs.)